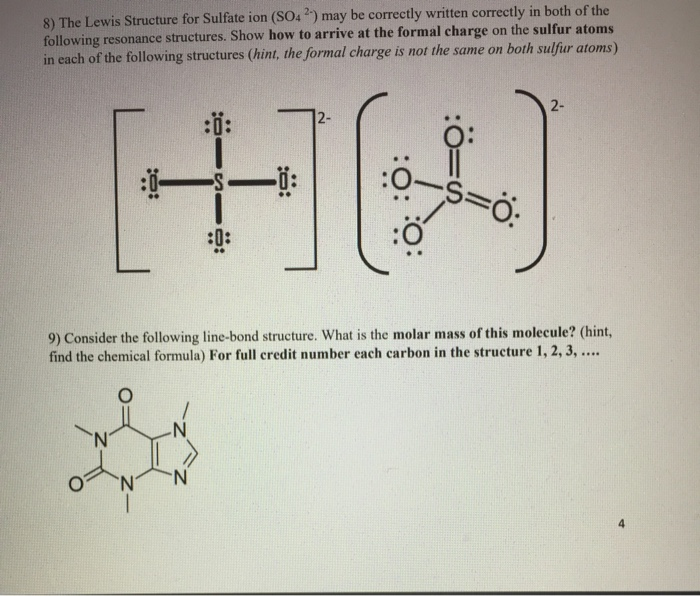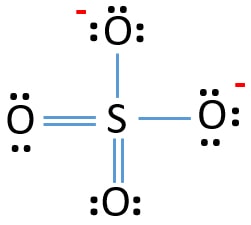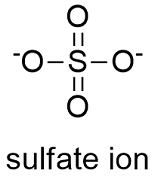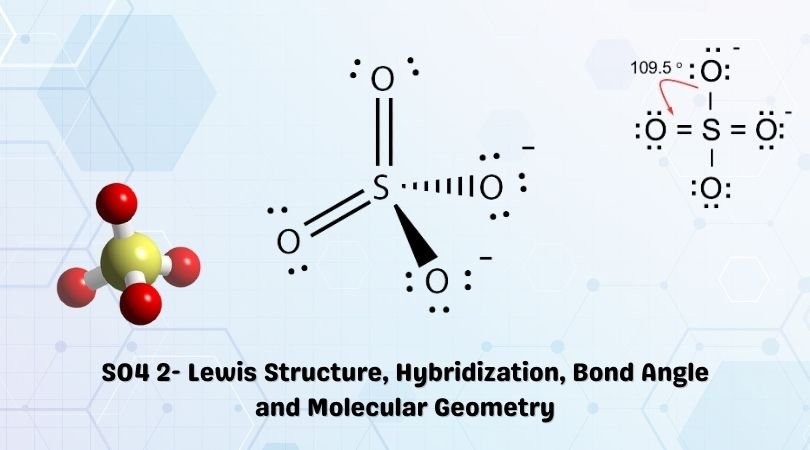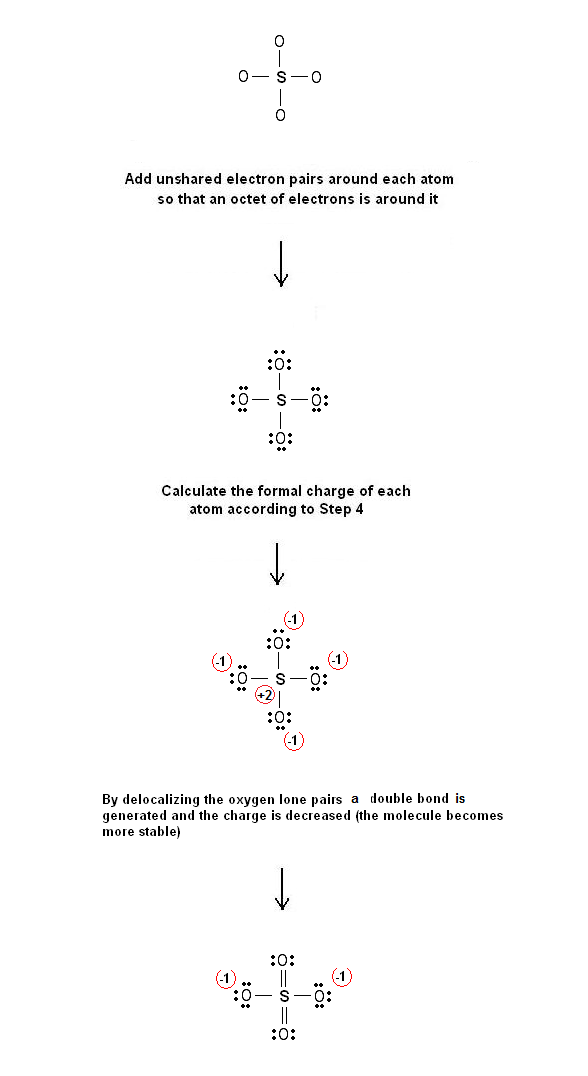
SOLVED:What is wrong with this Lewis structure? SO4 There are too many electrons There is nothing wrong with this Lewis structure: The S atom does not have an octet: The 0 atoms

Sulfate Anion, Chemical Structure. 3D Rendering. Atoms are Represented As Spheres with Conventional Color Coding: Sulfur (yellow Stock Illustration - Illustration of epsom, scientific: 188424839
![Aluminum Potassium Sulfate Dodecahydrate [AlK(SO4)2.12H2O] Molecular Weight Calculation - Laboratory Notes Aluminum Potassium Sulfate Dodecahydrate [AlK(SO4)2.12H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/12/aluminum-potassium-sulfate-dodecahydrate-molecular-weight-calculation.jpg)
Aluminum Potassium Sulfate Dodecahydrate [AlK(SO4)2.12H2O] Molecular Weight Calculation - Laboratory Notes
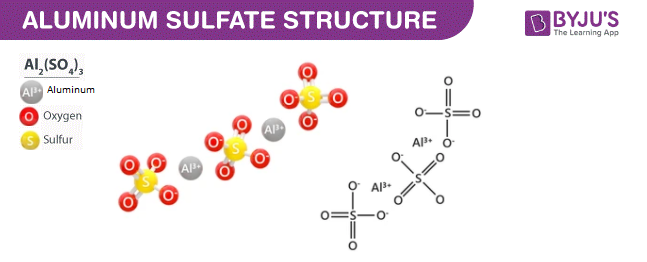
Aluminium Sulphate (Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>) - Structure, Preparation, Properties , uses and FAQs of Aluminium Sulphate (Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>)

Subscripts, Superscripts and Coefficients. Al 3 (SO 4 ) 2 coefficient subscripts superscripts ALUMINUM SULFATE SO 4 3- Al ppt download

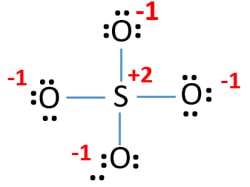
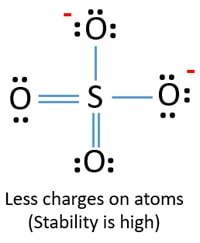
- Structure, Properties, Preparation, Uses Sulphate [SO4](2-)- Structure, Properties, Preparation, Uses](https://cdn1.byjus.com/wp-content/uploads/2019/08/Sulfate-ion.png)
![Atom labelling scheme for RE2[W2O3(SO4)6] (RE = Sm-Gd, Ho). Each W... | Download Scientific Diagram Atom labelling scheme for RE2[W2O3(SO4)6] (RE = Sm-Gd, Ho). Each W... | Download Scientific Diagram](https://www.researchgate.net/profile/Ulf-Betke/publication/264762292/figure/fig15/AS:646728147537920@1531203414400/Atom-labelling-scheme-for-RE2W2O3SO46-RE-Sm-Gd-Ho-Each-W-atom-is-surrounded.png)